
DEGME use survey
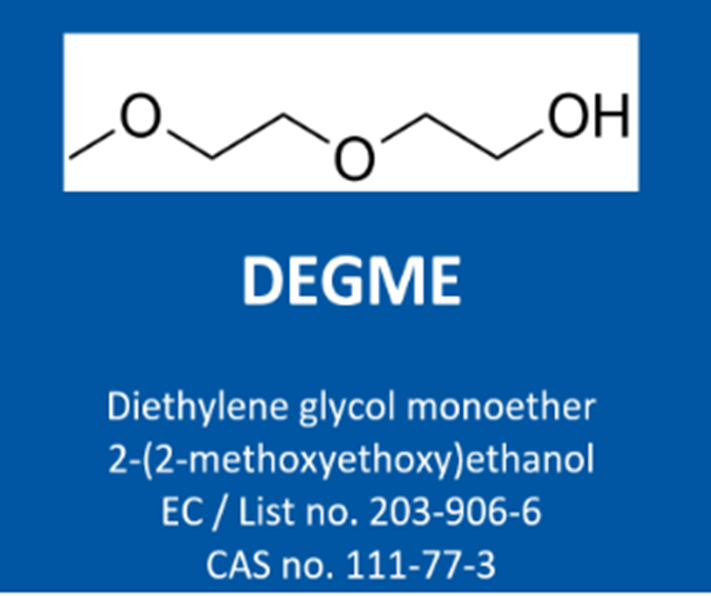 DEGME (Diethylene glycol monomethyl ether; CAS 111-77-3) has been classified for years as a reproductive toxicant Category 2 for developmental toxicity, where the generic concentration limit (GCL) for classification of 3% applies. But with the Adaptation to Technical Progress (ATP) 18, DEGME is since 1 December 2023 classified as H360D Cat 1B for developmental toxicity with a specific concentration limit (SCL) for classification of 3%.
DEGME (Diethylene glycol monomethyl ether; CAS 111-77-3) has been classified for years as a reproductive toxicant Category 2 for developmental toxicity, where the generic concentration limit (GCL) for classification of 3% applies. But with the Adaptation to Technical Progress (ATP) 18, DEGME is since 1 December 2023 classified as H360D Cat 1B for developmental toxicity with a specific concentration limit (SCL) for classification of 3%.
As the principles of Responsible Care are followed within OSPA, a specific charter is in place (Glycol Ethers Charter – Glycol Ethers Online (glycol-ethers.eu) for glycol ethers with a reproductive toxicity cat 1B classification. This charter states that the use of such substances should be limited to industrial applications, for which no substitute has been found so far and which are used under strictly controlled conditions. As a consequence, both direct customers and indirect customers via distributors have to declare their specific use and the control measures they have in place and indicate that they don’t have an alternative.
As a first step in the introduction of this OSPA charter for DEGME, a short survey was done to identify the uses and the control measures. However, it was determined that an online annual survey was a necessity to ensure proper monitoring of the uses and tonnage information applicable throughout the lifecycle of the substance. Penman and their IT service was selected for this task. The rollout of the online survey will be in June. Each EU manufacturer, distributor and user of DEGME will receive a link to fill in the survey. The data analysis will be done by Penman in a confidential manner, and only collated anonymised data will be given to OSPA for the purpose of ensuring that all uses, and relevant exposure scenarios are reported to ECHA to allow their continued use.
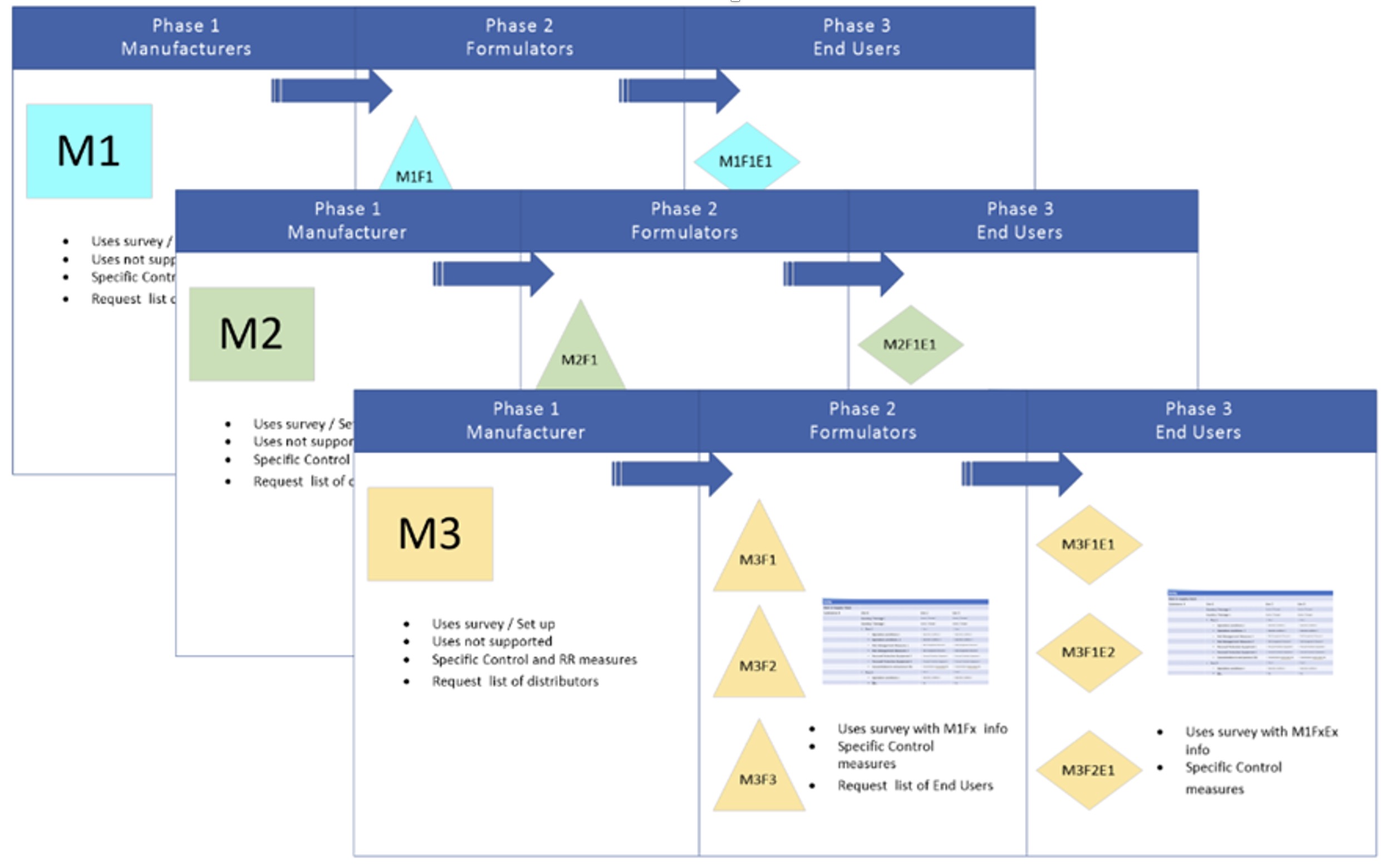 |
The purpose of the survey is to help the manufacturers and downstream users comply with the legal requirements of the EU chemical control legislation REACH and support the entire supply chain. The European Chemicals Agency (ECHA) have an increased focus on exposure issues and are demanding more information from registrants. This information will also be used to refine the OSPA charter with a specific annex to reflect that the RMM for DEGME may not need to be as onerous as for the other reprotoxic glycol ethers due to its low potency. |