
OSPA Launches DEGME Data Initiative to Enhance Supply Chain Safety
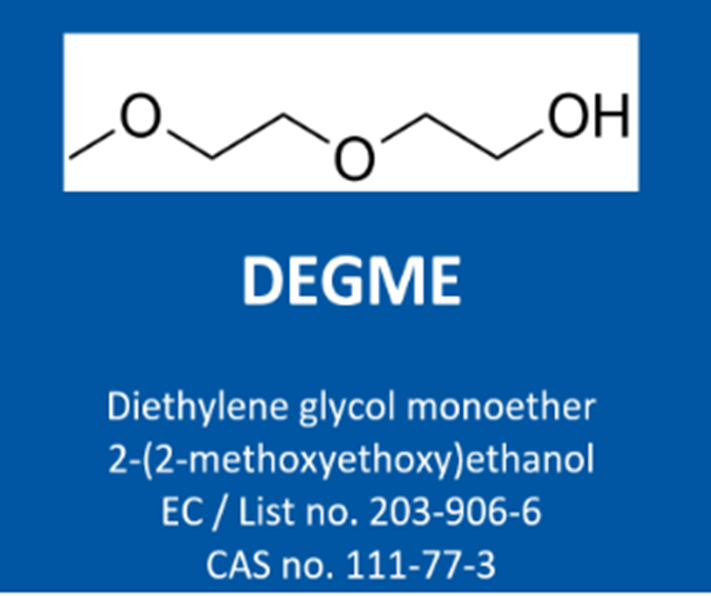
To meet the demands of hazard communication across the supply chain, the Oxygenated Solvents Producers Association (OSPA) has initiated a new project to close critical data gaps on Diethylene Glycol Monomethyl Ether (DEGME; CAS# 111-77-3). Following the recent reclassification of DEGME as a Category 1B reproductive toxicant, stricter regulatory measures will take effect on December 1, 2023. These include a specific concentration limit (SCL) of 3%, restricting use to professional applications only. This updated classification reflects rising concerns over DEGME’s potential developmental hazards and calls for updated compliance strategies.
To support industry stakeholders in adapting to these changes, OSPA has commissioned the consulting firm Penman to develop a comprehensive survey. This survey, scheduled to go live in late September 2024, aims to create transparent information exchange among manufacturers, formulators, and downstream users. The European Chemicals Agency (ECHA) has increased its focus on exposure data, demanding more detailed insights throughout the supply chain. Through targeted data collection, OSPA will refine its charter for DEGME. Given DEGME’s relatively lower potency compared to other reproductive toxic glycol ethers, these findings may help to streamline risk management measures (RMM) and ease regulatory burdens for the industry.
This initiative invites all EU manufacturers, distributors, and users of DEGME to participate. The data collected will be anonymized by the third-party consultant and serve as a foundation for future regulatory and safety measures. Through this proactive approach, OSPA is not only contributing to regulatory relief and sustainable use of DEGME but also reinforcing compliance along the supply chain and demonstrating the industry’s long-term commitment to safety and environmental responsibility.
HSE Considers Reclassification of 2-Butoxyethanol, OSPA Prepares Formal Response
The UK Health and Safety Executive (HSE) is currently reviewing a proposed reclassification of 2-Butoxyethanol, also known as Butyl Mony Glycol Ether (EGBE), from a Category 4 to a Category 3 hazard for acute inhalation toxicity. As part of this review, the HSE initiated a 60-day public consultation, which concluded on December 6, 2024. The Oxygenated Solvents Producers Association (OSPA) has thoroughly examined the “MCL Report for 2-butoxyethanol; ethylene glycol monobutyl ether (EGBE)” and has raised concerns regarding the proposed reclassification to Category 3. OSPA argues that the scientific evidence does not support this reclassification. In response, OSPA has prepared and submitted a detailed statement addressing these concerns within the stipulated timeframe.
EGBE is a widely used solvent integral to many industrial processes. Its current classification in the EU is based on extensive studies, primarily conducted with rats, which form the basis for assessing its health impacts. However, OSPA contends that a reclassification to Category 3 would not accurately reflect the risks posed to humans. Research has indicated that both humans and guinea pigs show greater resilience to the hemolytic effects observed in rats, suggesting that rat-based studies may not directly apply to human health risk assessment.
Recent findings, including a new OECD 433 study on guinea pigs, highlight these differences and question the applicability of rat models for human health predictions in the case of EGBE. This evidence underscores the importance of species-specific responses when evaluating the risk of solvents like EGBE. A recent study found no LC50 and reported a NOEC of ≥2.25 mg/L, indicating a low acute inhalation hazard.
The reclassification decision carries significant implications across industries, including the potential for EGBE to be classified as a Seveso substance. However, it is reassuring to note that the transport classification will remain unaffected by this reclassification. Observers are watching closely to see how HSE will weigh the latest scientific insights and stakeholder contributions in its final determination.